Abstract
Introduction:
Daratumumab (Dara) is the first CD38-directed monoclonal antibody approved for the treatment of Multiple Myeloma (MM). Dara is thought to have pleiotropic mechanisms of activity, including antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP). We focused our attention on potential mechanisms of enhancing macrophage (Mφ)-mediated ADCP and have highlighted that exposure of MM cells to low doses of Cyclophosphamide (Cy) led to a secretory response, which greatly augments Mφ-induced ADCP of Dara-coated MM cells1. Here, we sought to confirm this phenomenon in MM and establish the in-vivo relevance of these findings as seen in newly diagnosed MM patients enrolled in CyBorD-DARA phase 1b clinical trial (NCT02951819).
Materials and Methods:
Bone Marrow (BM) and peripheral blood (PB) samples were collected in EDTA vacutainers from newly diagnosed MM patients recruited to the CyBorD-DARA clinical trial both prior to- and 24hrs after Cy (150-300mg/m2) treatment. Total lymphocyte, monocyte and neutrophil counts were quantified by flow cytometry. Mononuclear cells were prepared and analysed by 8-colour flow cytometry to quantify monocyte, lymphocyte and plasma subsets as well as determine changes in surface expression profiles following treatment. As previously described, MM cells were conditioned with low-dose Cy for 24hrs. Media containing chemotherapy agents were removed and replaced with fresh media for 24hrs (TCM)1. THP-1 Mφ were conditioned with TCM for up to 48hrs and either characterised or incubated at 2:1 effector target ratio with CFSE labelled MM1S cells ± Cytochalasin D for 18hrs in the presence or absence of Dara/Isotype control. The % of Dara-specific tumour cell clearance was calculated to determine if the mechanism of tumour cell clearance was mediated by Mφ-mediated ADCP.
Results:
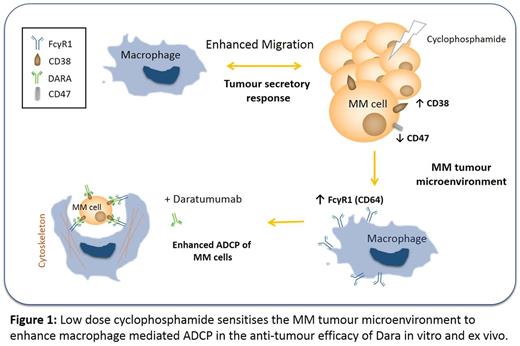
In patients receiving Cy for 24hrs, total BM and PB neutrophil numbers were increased (P<0.01, n=11), lymphocyte numbers were decreased (P<0.01, n=11) while monocyte numbers remain unchanged (P>0.05, n=11). More specifically, an increase in CD19+ B cells (P<0.05, n=6) and a decrease in natural killer cells (NK) (P<0.05, n=6) were observed. Interestingly, the number of Mφ precursor monocytes did not change following Cy treatment in both BM and PB of these patients. As innate immune cells are essential in immune surveillance, coupled with our finding that NK cells are reduced, this highlights a potential role for Mφ in Cy induced anti-tumour activity. Additionally, data previously presented highlighted that exposure of Mφ to TCM from Cy treated MM cells significantly enhanced % of Dara-specific clearance of MM cells) p<0.01). We also confirmed ADCP and ADCC as a likely mechanisms of tumour cell clearance in vitro.
Furthermore, we observed a marked reduction in expression of the "don't eat me" antigen, CD47, on chemotherapy-treated MM cells in vitro, which we have now confirmed in MM cells isolated from PB and BM of patients (n=2) following Cy treatment, which may enhance phagocytosis. We also observed increased expression of other targetable markers including BCMA (p<0.05), SLAMF7 (p=0.053), PD-L1 (p<0.001) and PD-L2 (p<0.001) in both RPMI8226 or MM1S MM cell lines following low dose Cy treatment in vitro. To date, we can confirm similar findings for both BCMA (p<0.05, n=5) and SLAMF7 (p<0.05, n=3) in patient samples. Furthermore, our in vitro observation of induced CD64 Fc gamma receptor expression on conditioned Mφ following single-agent Cy treatment has been confirmed in Mφ isolated from both BM (P<0.05, n=11) and PB (P<0.01, n=11) of newly diagnosed MM patients following Cy treatment. This finding emphasizes the potential physiological relevance of our in vitro data, implicating Cy in the potentiation of Mφ mediated ADCP in Dara specific anti-tumour immunity.
Conclusions:
Observations in Mφ precursors isolated from patients' BM and PB following 24hrs Cy treatment, confirm our previous in vitro findings which suggest a role for Cy potentiation of Mφ mediated ADCP in anti-tumour efficacy of Dara. These findings provide a rationale for the efficacy observed to date when combining Cy with Dara in the ongoing CyBorD-DARA clinical trial. Additionally, increases in BCMA and SLAMF7 highlight the potential of other combination immune therapies to further enhance therapeutic efficacy in these patients.
1. Rigalou et al, Blood 2016 128:2101
Chiu: Janssen: Employment. Sasser: Janssen Pharmaceuticals: Employment. Ryan: Janssen: Research Funding. O'Dwyer: Onkimmune Ltd: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; GlycoMimetics Inc: Research Funding; Janssen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal